HARD
12th CBSE
IMPORTANT
Earn 100
An alkene (Mol. formula ) on ozonolysis gives a mixture of two compounds and . Compound gives positive Fehling’s test and also forms iodoform on treatment with and . Compound does not give Fehling’s test but forms iodoform. Identify the compounds and . Write the reaction for ozonolysis and formation of iodoform from and .

Important Questions on Aldehydes, Ketones and Carboxylic Acids
MEDIUM
12th CBSE
IMPORTANT
Which of the following compounds is most reactive towards nucleophilic addition reactions?
MEDIUM
12th CBSE
IMPORTANT
Compound
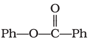 can be prepared by the reaction of _____.
can be prepared by the reaction of _____.MEDIUM
12th CBSE
IMPORTANT
Cannizzaro’s reaction is given by _____.
MEDIUM
12th CBSE
IMPORTANT
Which product is formed when the compound
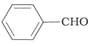 is treated with
is treated withconcentrated aqueous solution?
EASY
12th CBSE
IMPORTANT
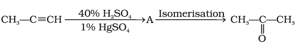
The structure of and type of isomerism in the above reaction are respectively:
HARD
12th CBSE
IMPORTANT
An aromatic compound (Molecular formula ) gives positive test. It gives a yellow precipitate of compound on treatment with iodine and sodium hydroxide solution. Compound does not give Tollen’s or Fehling’s test. On drastic oxidation with potassium permanganate it forms a carboxylic acid (Molecular formula ), which is also formed along with the yellow compound in the above reaction. Identify and and write all the reactions involved.
HARD
12th CBSE
IMPORTANT
Write down functional isomers of a carbonyl compound with molecular formula . Which isomer will react faster with and why? Explain the mechanism of the reaction also. Will the reaction lead to the completion with the conversion of whole reactant into product at reaction conditions? If a strong acid is added to the reaction mixture what will be the effect on concentration of the product and why?
HARD
12th CBSE
IMPORTANT
When liquid is treated with a freshly prepared ammoniacal silver nitrate solution, it gives bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogen sulphite. Liquid also forms a white crystalline solid with sodium hydrogen sulphite but it does not give test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
